Trelleborg Healthcare and Medical
Trelleborg Healthcare & Medical
Bring your Medical Devices to Market Faster.
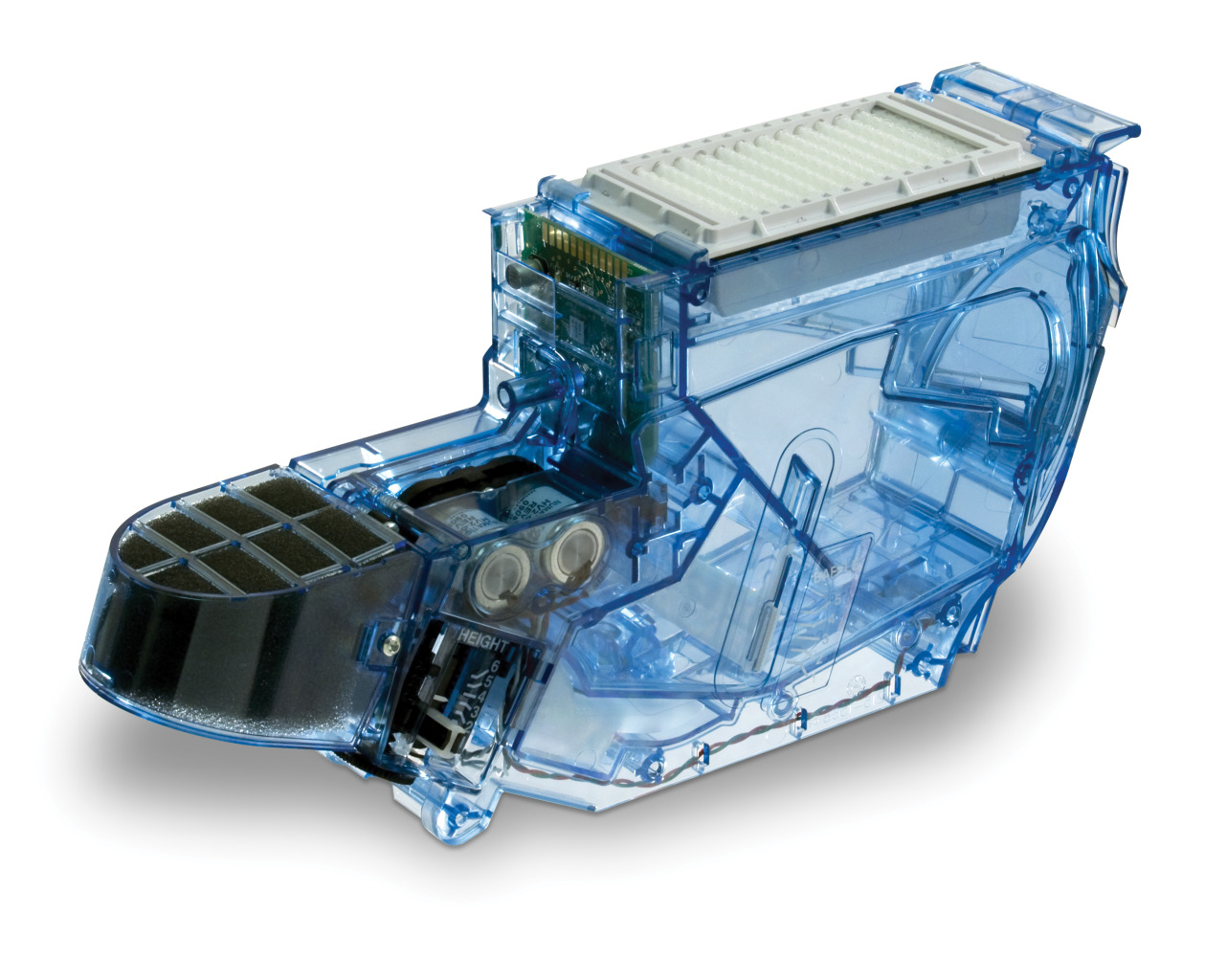
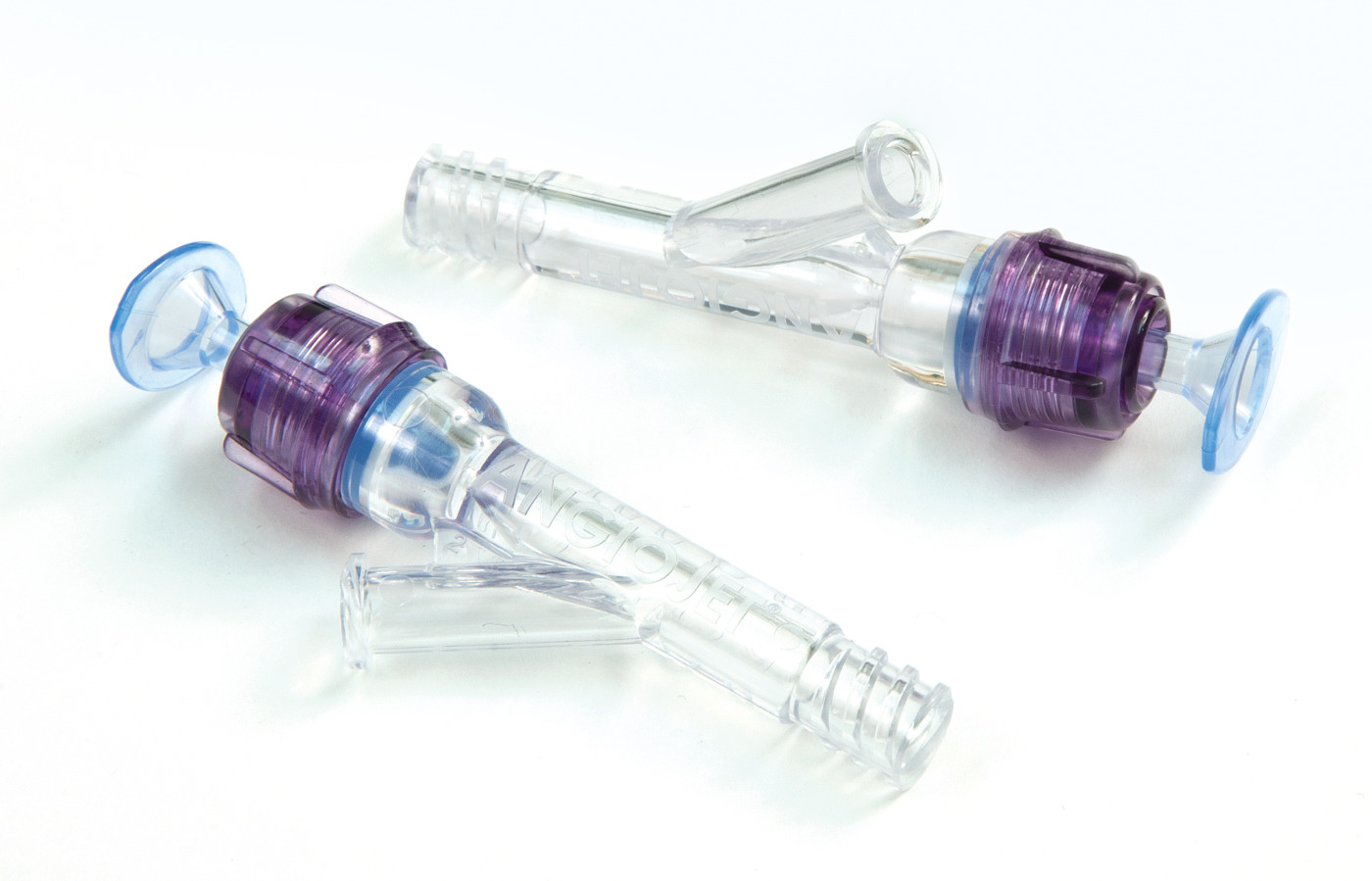
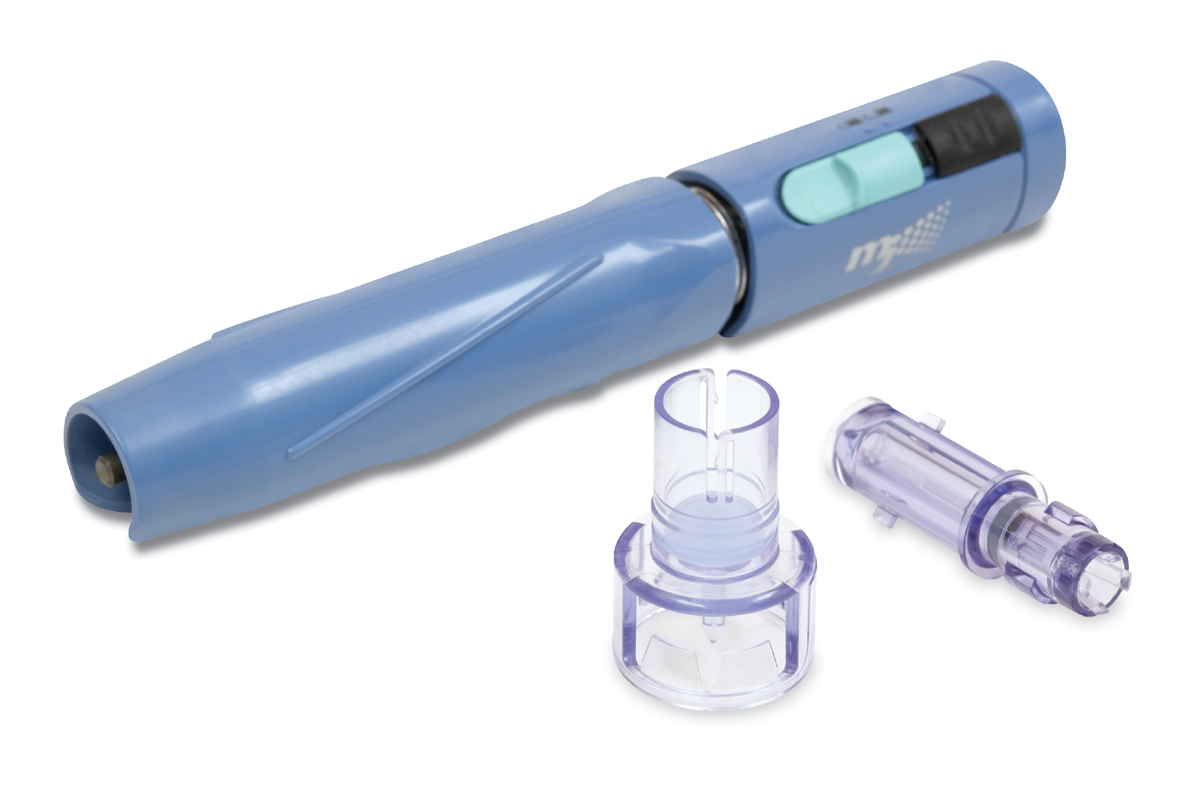
Trelleborg Healthcare & Medical is one of the world’s leading developers, manufacturers and suppliers of precision seals, bearings, and custom-molded polymer components for demanding medical device, biotech, and pharmaceutical applications. By forming collaborative partnerships, we help design, develop, manufacture, and bring to market innovative engineered solutions to meet the most challenging needs of healthcare and medical device companies.Trelleborg has expanded its capabilities and offerings to customers with the acquisition of the US-based Minnesota Rubber & Plastics (MRP). Headquartered in Plymouth, Minnesota, MRP is a leading manufacturer of elastomeric and thermoplastic components and a system provider for technically demanding medical device applications. From high-tolerance medical devices, diagnostic components and assemblies to final sterile-packaged devices, we specialize in the most demanding applications using medical-grade elastomers, liquid silicone rubber (LSR) and thermoplastics. Capabilities include materials science and formulation, fully functional product prototyping, and operational excellence and supply chain consolidation.
Trelleborg Healthcare & Medical now comprises 41 manufacturing facilities in the Americas, Europe, and China, 61 Customer Solution Centers, 10 R&D Centers, five Logistics Centers, four ServicePLUS Centers, an Innovation Center, and a Rapid Development Center. We serve healthcare and medical customers globally with capabilities including silicone and thermoplastic molding and overmolding, extrusion, sheeting, dipping, coating, plastic injection molding, multicomponent manufacturing, micro-molding, automation, drug-eluting technologies, engineered coated fabrics, and other technologies.
With our global footprint, we help customers innovate their medical device, biotech, and pharmaceutical applications, including:
- Biopharmaceutical
- Cardiovascular
- Cell Therapy
- Diabetes
- Diagnostics
- Sterile Fluid Connectors
- Structural Heart
- Surgical Instruments
- Targeted Drug Delivery
Find the best material fit for your application through our:
- Market expertise
- Accessible experts
- On-premises advanced materials lab
- Ever-expanding library of thousands of proprietary materials
- Hundreds of custom elastomer formulations tested to standards USP Class VI, USP, Ph. Eur. 3.2.9, and ISO 10993
New Product Design + Development
We have dedicated and highly experienced product design engineers ready to collaborate with you by providing product design assistance to ensure the successful and timely launch of differentiated solutions. Our product design engineers conceptualize and evaluate ideas by combining science and technology to create value. Their development role is facilitated by digital tools that allow them to visualize and analyze new designs, materials, and products.
Our comprehensive design process includes:
- Mechanical design and DFM review
- Lean Six Sigma best practices
- Unified project management
- Materials analysis; specialty elastomer compounds
- Design and development
- Prototyping services
- FEA and mold-flow analysis
- 7 Stage NPD Process from opportunity assessment to production control manufacturing, testing, and assembly
- Functional and leak testing
As a manufacturer of high-precision components and assemblies, FDA Class 1, 2, and 3 products, Trelleborg Healthcare & Medical specializes in finding solutions to tough applications which require the molding and assembly of close tolerance components. We offer a wide range of technical services and production capabilities from Design for Manufacturability (DFM) to Lean Six Sigma best practices, producing close-tolerance injection molded components, assemblies, and final sterile packaging.
Our range of processing capabilities extends from thermoplastics to conductive elastomers including, LSR and gum stock silicones, or high consistency rubber (HCR).
Robust quality and processes
Your custom-molded rubber and thermoplastic components can be manufactured and supported at any one of several locations we have around the world. Our worldwide manufacturing facilities operate under strict ISO guidelines.
- FDA registered sites in USA, Switzerland, and China
- ISO 13485, ISO-TS 16949 and ISO 9000 certifications
- ISO Class 7 and 8 cleanroom manufacturing
- Environmental Management System registered to the International Standards Organization series ISO-14001
- Drug Master Files
- Many materials are compliant with ISO 10993, USP Class VI, and U.S. Food and Drug Administration requirements
- Mature Lean Six Sigma program
Our global manufacturing and supply chains put you closer to your customers. Across our worldwide operations, we demonstrate a commitment to excellence and performance by offering our customers greater value and quality in the products we sell. Our global resources provide comprehensive service, support, and sourcing options.
Contact us today to learn more
Corporate Headquarters
Trelleborg Healthcare & Medical
1100 Xenium Lane N.
Minneapolis, MN 55441
Phone 1-800-927-1422
Fax (952) 927-1470
E-mail: [email protected]
Web: www.mnrubber.com