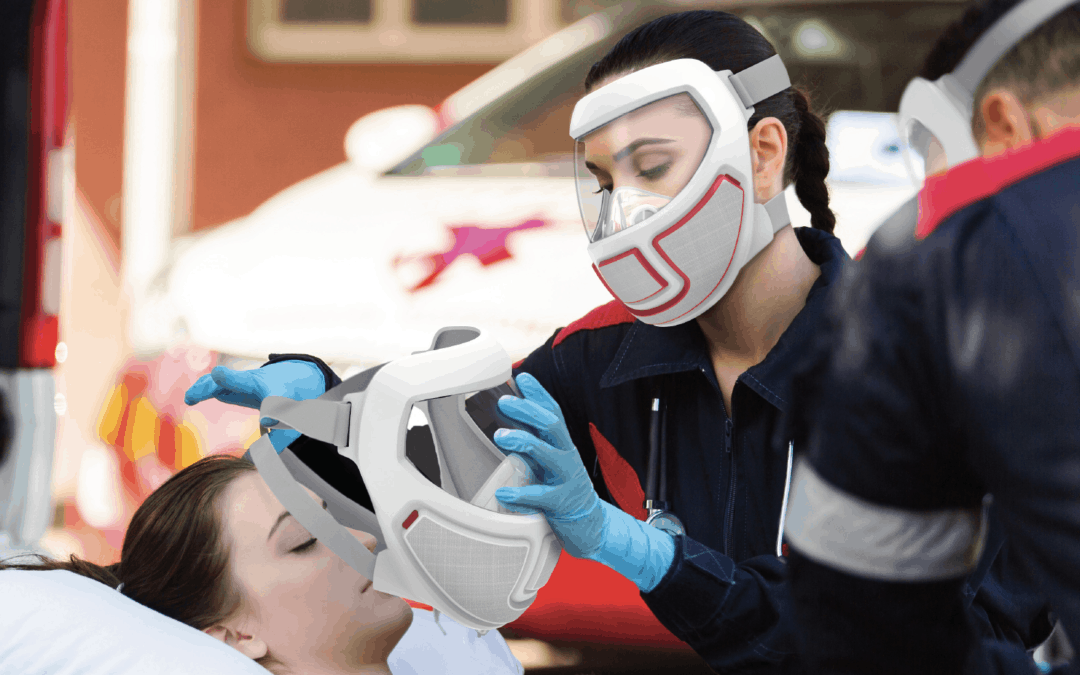
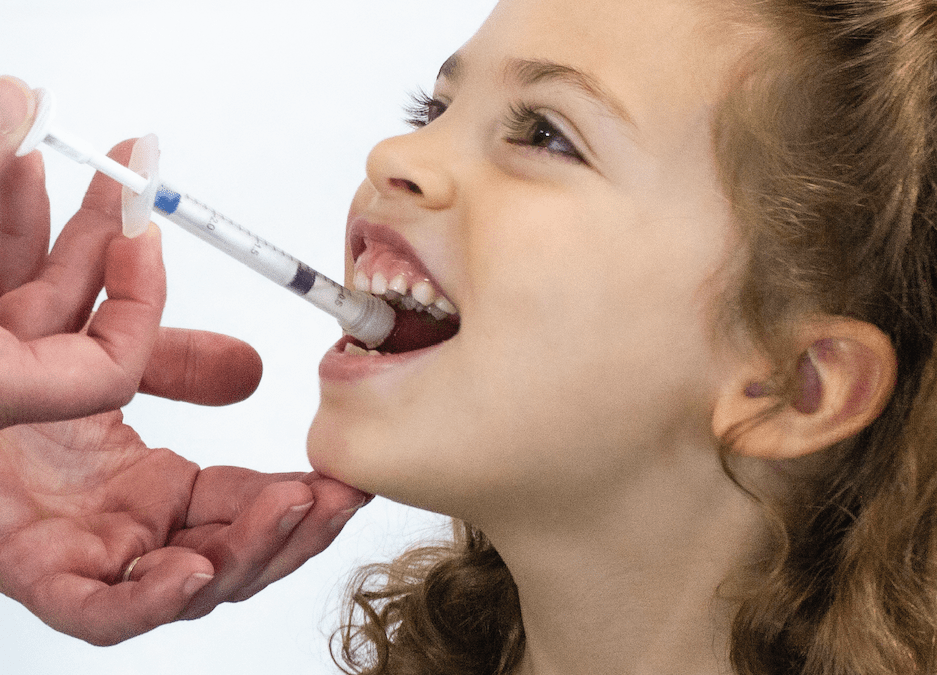
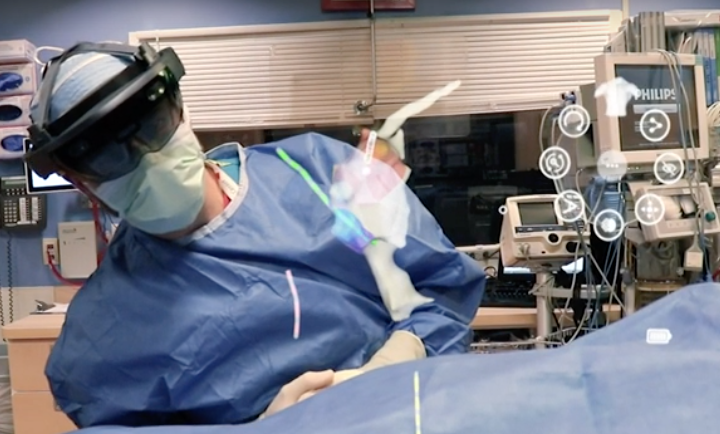
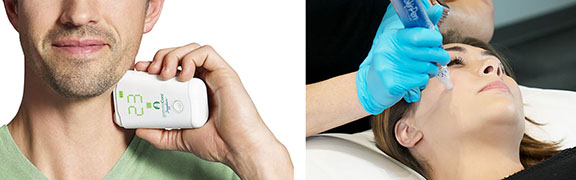
HS Design, Inc. an Award-Winning Design Firm dedicated to Medical, Life Science and Consumer Health markets, announced today that it has once again, re-affirmed its commitment to quality by maintaining its ISO 13485:2016 certification following a successful inspection conducted by the British Standards Institute (BSI).
HS Design, Inc. an Award-Winning Design Firm dedicated to Medical, Life Science and Consumer Health markets, announced today that it has once again, re-affirmed its commitment to quality by maintaining its ISO 13485:2016 certification following a successful inspection conducted by the British Standards Institute (BSI). This achievement indicates that HSD has met the highest standards required of quality management in the medical devices and life science industries.
“Although we have been certified for several years, maintaining the ISO certification is a great accomplishment as the bar keeps rising,” said Michael Quinn, VP of Design & Engineering. “By working with BSI on ISO 13485:2016 we make sure we are kept accountable to world-class quality. We call it “Focused Innovation”, quality without compromising design, but by embracing all the aspects that go into good design.”
About HS Design, Inc. (HSD)
HSD is an ISO 13485 certified medical design firm with over 40 years experience specializing in solving complex problems in life science, medical, surgical, consumer healthcare systems, including high volume consumer medical devices and disposables.
Our capabilities include ISO 62366-compliant Design Research, Human Factors, Industrial Design, UI/UX Design, Mechanical Engineering, Electrical Engineering, Software Engineering, Rapid and Pilot Prototyping, and Manufacturing Transfer. We partner with global Contract Manufacturing teams to deliver seamless integration of full-service product development aimed at rapid design transfer and production.
HSD has been integrating insight, experience, and innovation with user needs & client core competencies to develop products that improve the human experience.