AIM Plastics Inc.
AIM understands that medical manufacturing is constantly evolving. In order to compete in today’s market you need to rely on a company that has the experience to bring your design to market without compromise. This experience is why AIM Plastics is considered a premiere medical plastics manufacturer. AIM collaborates with some of the medical industry’s largest companies looking to fulfill mid to high volume medical manufacturing needs. For more information on AIM Plastics CLICK HERE
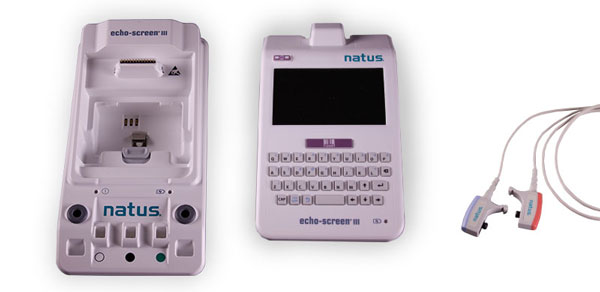
Services Offered
PRECISION MOLD MAKINGAIM’s in-house mold design and tooling construction offers significant benefits. Our corporate roots stem from highly skilled mold designers....




